Vaccine Safety
Vaccines are held to the highest standards of safety. Before they are approved for use in Canada, they must be shown through multiple clinical studies to be not only effective in preventing disease but also safe to use. Canada's long-standing vaccine safety system ensures that vaccines marketed and used in Canada are as safe as possible. Health authorities in Canada take vaccine safety very seriously.
Vaccines undergo a series of testing before they are approved for use. Most common adverse events associated with a vaccine are identified in studies before the vaccine is licensed, but rare adverse events may not be detected in these studies. Therefore, the Canadian vaccine safety system continuously monitors for possible adverse events after a vaccine is licensed. When millions of people receive a vaccine, less common adverse events that were not identified earlier may emerge.
If a link is found between a possible adverse event and a vaccine, public health officials take appropriate actions. These include determining if the recommendation for using the vaccine should change.
Although vaccines, like any medication, can have associated adverse events, not immunizing also involves risk. Not immunizing puts oneself and others who come into contact with him or her at risk of contracting diseases vaccines are meant to prevent, which can be serious, or even deadly. Even though many of these diseases have become rare in this country (thanks in large part to vaccines), they still commonly occur elsewhere around the world and can be brought into Canada, putting unvaccinated individuals at risk.
Vaccine Safety – Immunize Canada
Adverse Events Following Immunization
An Adverse Event Following Immunization (AEFI) is any untoward medical occurrence in a vaccinee that follows immunization. It may be any unfavourable and/or unintended sign, abnormal laboratory finding, symptom or disease. The vaccine or its administration may not necessarily have been the cause.
The Public Health Agency of Canada’s (PHAC) Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) monitors AEFIs from across the country through local public health units. The surveillance system seeks to ensure the continued safety of vaccines in the Canadian market.
The most common AEFIs are mild, such as redness and swelling where the shot was given, and go away within a few days. If your child experiences a reaction at the injection site, you can use a cool, wet cloth to reduce redness, soreness, and swelling.
Serious AEFIs, such as severe allergic reaction, are very rare, and doctors and clinic staff are trained to deal with them. After vaccination, if you observe any reaction that concerns you, consult a health care professional.
Reporting requirements in Manitoba
In accordance with Section 59 of The Public Health Act, health care providers are to report a reportable AEFI within seven days of becoming aware of the AEFI. Furthermore, health care providers should report a serious AEFI within one business day, which can be by telephone, followed by the complete written report within 72 hours.
A reportable AEFI is an event that:
- is temporally associated with a vaccine AND
- has no other clear cause at the time of reporting
Of particular interest are AEFIs that are serious, unexpected and/or of special interest. But ALL AEFIs that meet (1) and (2) above should be reported, unless they are only mild local reactions that are not overly concerning to the vaccine recipient.
Please see the User Guide for the Completion and Submission of the AEFI Reports for definition of serious AEFI.
An AEFI is considered “unexpected” if either of the following criteria is met:
- Is not listed in the most current Health Canada-approved product monograph for vaccines marketed in Canada
- Listed in the product monograph but is different in nature, severity, frequency, specificity or outcome.
Information contained in the User Guide for the Completion and Submission of the AEFI Reports will provide immunization providers with direction for how to correctly complete and submit either in PHIMS or by using the PDF reporting form. For providers opting to use the PDF form, there are instructions at the bottom of the last page for how and where to submit the completed form.
Reporting fatal outcomes
Report death outcomes that meet the following criteria:
- Death occurred within 30 days of vaccination AND
- The antecedent AEFI or medical condition that led to the demise itself is reportable. If no antecedent AEFI is identified, the death itself is the AEFI and should be reported as such if occurred within 30 days.
How AEFIs are monitored
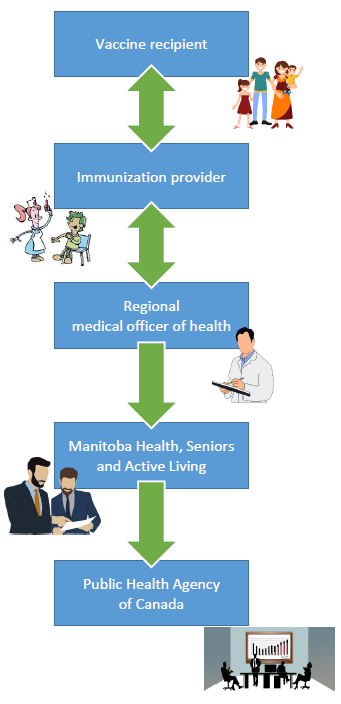
In Manitoba, AEFI reports are received at the health regions from multiple sources: physicians, nurses, pharmacists and public health.
Recommendations are made by the regional Medical Officer of Health, who conveys it to the vaccine recipient’s immunization provider. The data are shared with Manitoba Health (Health), which reviews them before forwarding to PHAC. Health may, in coordination with the concerned RHA and in consultation with other local experts as well as with PHAC, perform any further investigation that may be required.
Personnel in PHAC’s Vaccine Safety Section screen all submitted reports, ensure they are entered into the CAEFISS database and coded using standard international coding terminology. Reports are monitored with special attention to serious or unusual events that could signal a concern regarding vaccine safety.
This reporting process is illustrated below:
Vaccine Injury Support Program (VISP)
The Public Health Agency of Canada has established a Vaccine Injury Support Program (VISP) in partnership with provinces and territories. While serious side effects are very rare, this program ensures anyone who experiences a serious side effect or injury because of the COVID-19 vaccine will be able to seek financial support. The VISP is now accepting claims. People in Canada who have experienced a serious and permanent injury after receiving a Health Canada authorized vaccine, administered in Canada, on or after December 8, 2020 can submit a claim requesting financial support.
For more information on the VISP and how to submit your claim please visit: https://www.canada.ca/en/public-health/services/vaccination-children/safety-concerns-side-effects.html#a5 or vaccineinjurysupport.ca.
Manitoba Health Resources
For Health Care Providers
- User Guide for the Completion and Submission of the AEFI Reports
(November 2016) - MMRV-Febrile Seizure & RV-Intussusception
(February 2017)
Forms
- Reporting Form for Adverse Events Following Immunization (AEFI)
 - MHSU-2334 REVISED (fillable)
- MHSU-2334 REVISED (fillable)
(November 2016) - Brighton assessment tool (MS-Access file; right-click to download)
(October 2013) - Manitoba Reporting Form on Causality Assessment of Adverse Event Following Immunization (WHO)
 NEW
NEW
(June 2016) - Manitoba Reporting Form on Causality Assessment of Adverse Event Following Immunization (CISA)
 NEW
NEW
(June 2016)
Other Resources
- BC Centre for Disease Control – Immunization Manual: AEFI section
- Immunize Canada – Vaccine Safety
- World Health Organization – Global Advisory Committee on Vaccine Safety
- Canadian Adverse Events Following Immunization Surveillance System – Data reports and publications
Anaphylaxis (including Management)
Communicable Disease Control (CDC)
Public Health
Manitoba Health
4th Floor - 300 Carlton St.
Winnipeg MB R3B 3M9 CANADAHealth Links – Info Santé
204-788-8200 or 1-888-315-9257


